
Autoscribe LIMS Blog
Items related to:


Regulatory
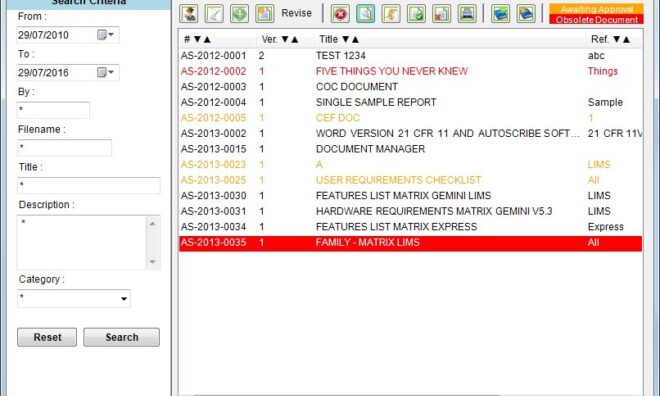
ISO 17025 Documentation within a LIMS Solution using Matrix Gemini LIMS - Part 2
Part 2 - Corrective and Preventative Actions (CAPA)
29th November 2016


Regulatory
ISO 17025 Documentation within a LIMS Solution using Matrix Gemini LIMS - Part 1
Part 1 - Document Management
25th August 2016
Applications
Genuine System Configurability and its Impact on Global LIMS Projects
In my 30 years in the LIMS business I have seen many global LIMS projects fail or, at best, be criticized heavily by the users of the delivered systems.
26th July 2016
Regulatory
Environmental Microbiology Monitoring - Keeping the Public Safe
Ensuring Sterile/Hygienic Environments
26th July 2016

Regulatory
Matrix Gemini Stability Helps Meet FDA Compliance For Stability Testing of Pharmaceutical Products
22nd July 2016
Let‘s Talk
Find out how we can help maximize efficiency and minimize risk for your laboratory.
Follow Autoscribe
Let’s Talk
Ready to get started? Contact us today.
Let’s connect and we’ll arrange a Matrix LIMS demo.