
Autoscribe LIMS Blog
Items related to:




Regulatory
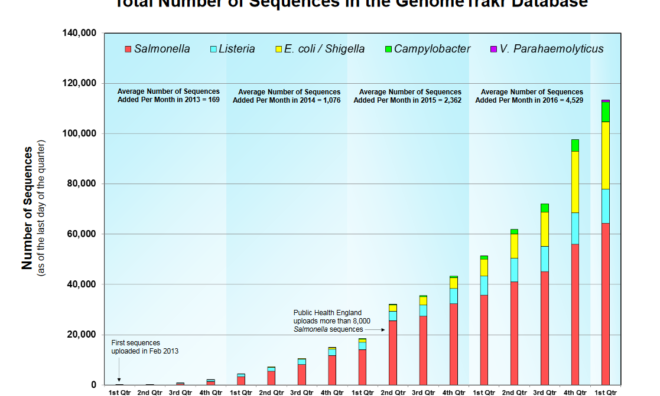
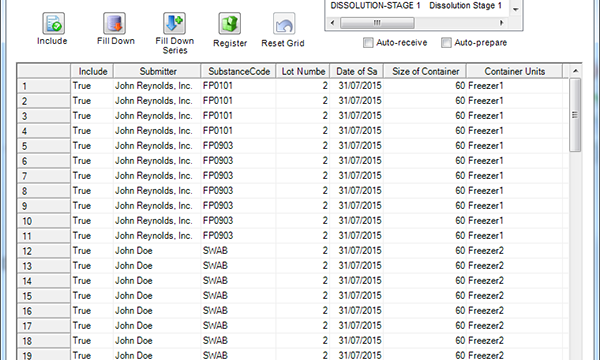
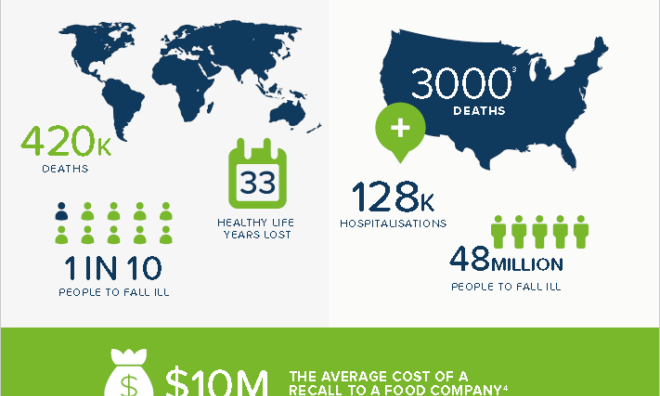
FDA Inspection Checklist Emphasizes "Robust" Environmental Monitoring Systems
25th January 2017
Let‘s Talk
Find out how we can help maximize efficiency and minimize risk for your laboratory.
Follow Autoscribe
Let’s Talk
Ready to get started? Contact us today.
Let’s connect and we’ll arrange a Matrix LIMS demo.