Blog: Applications
Testing the Limits in LIMS - Part 1
A two-part series covering what limits are and how to apply them in a LIMS.
6th April 2024

One of the many benefits of implementing a Laboratory Information Management System (LIMS) is the reduction, or elimination, of potential user error and the consistent application of standard operating procedures. Nowhere is this more important than in checking test results against predefined limits. These limits may refer to the test itself, for example it should not be possible to record a result of 15 for a pH measurement. Alternatively, they may refer to a product being tested where, for example, it is important to ensure results are compared to the correct product specification. A flexible LIMS will allow different types of limits to be defined to meet these different requirements, and ensure the checking occurs consistently. However, how these limits should be applied may be misunderstood and requires careful planning. This article therefore discusses different types of limits and how these can be applied within a LIMS.

Laboratories test a huge range of different types of samples. In a manufacturing environment raw material may need testing before release to manufacturing, where intermediates and final products will also require testing at various stages in the production process. For each test there can be limits applied which, at the most basic level, will indicate if the results are within the defined specification for the specific raw material, intermediate or final product. Limits like these are used in all manufacturing industries from pharmaceutical and medical devices to chemicals and food & beverage. Similarly in healthcare limits can be used to check if results are within acceptable ranges, and in environmental laboratories limits would be applied to the various contaminants in water samples.
Deriving limits and using them within the laboratory therefore forms an important part of both analytical testing and setting up the LIMS correctly. Different types of limits become especially important if the same test is used for different products, product grades or sample types. Again, a simple example of this would be a pH test that could be used in multiple circumstances. However, consider the example of a pharmaceutical product that is manufactured in multiple dosages or dosage forms; many of the tests will be the same but the acceptable results will be different depending on the dosage or dosage form. The situation can become even more complex if the test has a large number of analytes or components associated with it. An example of this would be detection of metals using Inductively Coupled Plasma (ICP) analysis where multiple different metals can be detected. However, the acceptable limits for the metals may depend on the sample or type of sample under test. It would be time consuming and inefficient to have to create and maintain the basic ICP test multiple times for each sample or sample type.
It is also important to note that the limits definition in LIMS must support ranges, lower values only or upper values only. For example, the result must fall between the defined upper and lower values to be within the limits, or the result must not be less than a specific value to be within the limits or the result must not be greater than a specific value to be within the limits. In the example of heavy metals in water the limits are likely to be defined as the result must not be greater than the specific values; whereas for the amount of active ingredient in a pharmaceutical the limits are likely to be defined as the result must be within the upper and lower values In the case where a test result is not a numerical value but instead a predefined response, such as an observational assessment, it must be possible to define which responses are within limits and which are not.
Various types of limits must be available within a LIMS to trigger different actions. Actions must include (in order of severity) the ability to fail the sample result, to give a warning, and to provide a notification to the user based on the sample result. These will explore limit types in more detail in part 2.
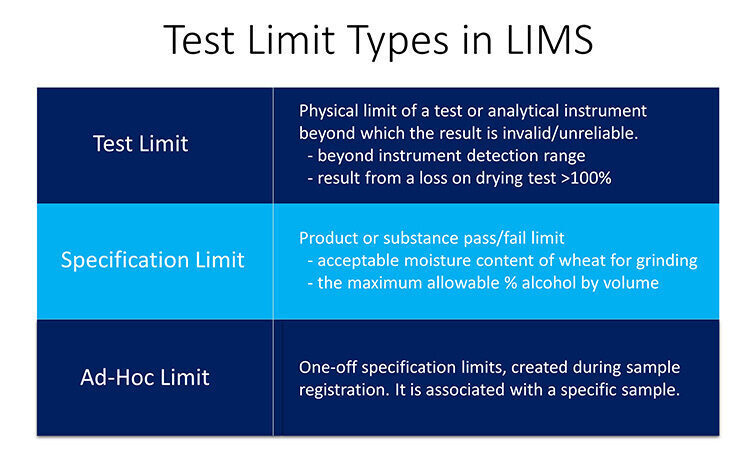
Test Limits
Test limits can be used for limits that must be applied irrespective of the context within which the test is being used. Generally, they are used to prevent irrational or impossible results being entered. It should not be possible to enter and record a pH value above 14 and the result of a loss on drying test should not be greater than 100% no matter what is being tested. Test limits can prevent such impossible results being recorded.
In a similar way some test results are only accurate within a range or results, and outside this range they become unreliable. A good example of this are results obtained from a test instrument which are towards the edge of the instrument’s capabilities, known as the instrument detection limit. Results below (or above) this limit are indeterministic. Results can either be discounted, or reported as below the instrument limit, as appropriate to the test. Another example of an analytical limit is that some tests will only provide valid results within a linear range, with results outside this range being unreliable. LIMS need to be able to apply a limit to these results, ensuring only the linear range is used for reporting purposes, in order that results are always handled in a deterministic way.
Specification Limits
Specification limits which may also be referred to as product limits or substance limits cover most of the limits used. The acceptable moisture content of wheat for grinding into flour, the range of white blood count values for adults, and the maximum allowable % alcohol by volume for a product to be defined as low alcohol are all examples of this type of limit.
Specifications for what seem to be similar, or even the same, product may change dependent on the customer or market, for example supermarkets may define specifications for different types of the same product that may help differentiate price and market. Local regulations or cultural factors may require different specifications for a pharmaceutical product. A LIMS must be able to easily cope with these types of differences.
A specialized type of specification limit is the grading limit. A product may be checked against its standard manufacturing specification. If the product fails the standard specification a grading specification could be used to see if it passes an alternative specification and be acceptable to a specific customer who would accept this wider specification limit. This can help improve efficiency by stopping problem batches being scrapped or having to be reworked. Alternatively, a batch that passes its manufacturing specification can be graded against a higher specification to see if it can be sold at a premium.
Other Limits
There are circumstances in which specific types of limits may be required. There may be a situation where a sample needs to be tested against a one-off set of specifications that are not defined in the system. It should be possible to create these ad-hoc for specific samples at the time of sample creation, where they will be associated with the sample and its associated tests. At result entry the result values will be assessed against these one-off specification limits. Where stability studies are used to assess the shelf life of pharmaceutical products, there may be a need for separate product limits that are applicable only to the stability study and again a flexible LIMS will allow for the definition and management of these type of specification.
Approval and Release of Test and Specification Limits
Test and specification limits are usually created within the LIMS by the LIMS administrator, who must also approve the limits. Once approved the limits are made available for use by all relevant users. Once a test (with any built-in limits) is attached to a sample this version of the test does not change, even if the limits are subsequently updated by the LIMS administrator. This mechanism ensures a correct historical record is kept of the test, and the limits which were in force at the time the test was applied to the sample. Version control of tests and their limits are a key part of managing laboratory data quality for audit purposes within ISO 17025/15189.
Summary
In part 1 we discussed what limits are, and the various types of limits. In part 2 we shall discuss how these limits are applied to a LIMS.