Blog: Applications
Pooled Sample Testing in LIMS
12th February 2024

Pooling samples and testing the resulting single sample can help laboratories improve productivity, reduce reagent use, and cut costs. Pool-testing can be used to increase throughput if testing resources are limited or during times of increased demand.
Sample pooling is used in many industries. In healthcare, for instance, it may be used for screening blood donations to ensure donated blood does not contain HIV or Hepatitis C and is safe to use. It is commonly used in Veterinary labs checking cattle herds for Bovine Virus Diarrhea in pooled serum, and Brucella testing in pooled milk samples. Flocks of birds may also be quickly checked for Avian Influenza Virus using pooled nasal or cloacal swabs.
A variation of sample pooling, or composite sampling, can be used to sample different materials and test them together in a single combined test, saving time and resources. For example, a contract lab needing to check for lead in a child’s toy can combine scrapes of the paint and other materials into a single sample rather than test each sub-component separately. The result is a pass or fail of the assay for the presence of lead for the entire toy.
Pooled samples can be particularly useful when a positive result means that the entire herd or flock must be culled. If a pooled sample tests positive the whole herd can be flagged with no need to test each individual animal. Pooled testing is especially useful for diseases where the incidence is relatively low. To gain the benefits from pooled testing it is important that the pool size is set correctly. This is particularly true when it is used in general population surveillance monitoring. During the Covid-19 epidemic there has been much debate about the use of pooled testing and what the size of the pool should be.
Pooling Techniques
Sample pooling is not effective if the rate of retest required is too high because, for example, the pool size has not been set correctly. In this case retesting quickly adds to cost, complexity, and time, wiping out the potential gains of sample pooling.
More complex pooling strategies, such as hierarchical pooling, exist as referenced in the paper ‘Sample pooling: burden or solution?’[1]. Hierarchical pooling describes a multi-step approach whereby if a sample pool fails you split it into two or more sub-pools and retest to find the failing sub-pool. The individual samples of this (smaller) sub-pool are then retested individually. Determining the size of the initial sample pool, and the retest sub-pool size is beyond the scope of this article, but is explained in detail in the referenced paper, and many other papers. However, once the pooling strategy has been defined it can be codified into a suitable LIMS to automate sample pooling and retest procedures.
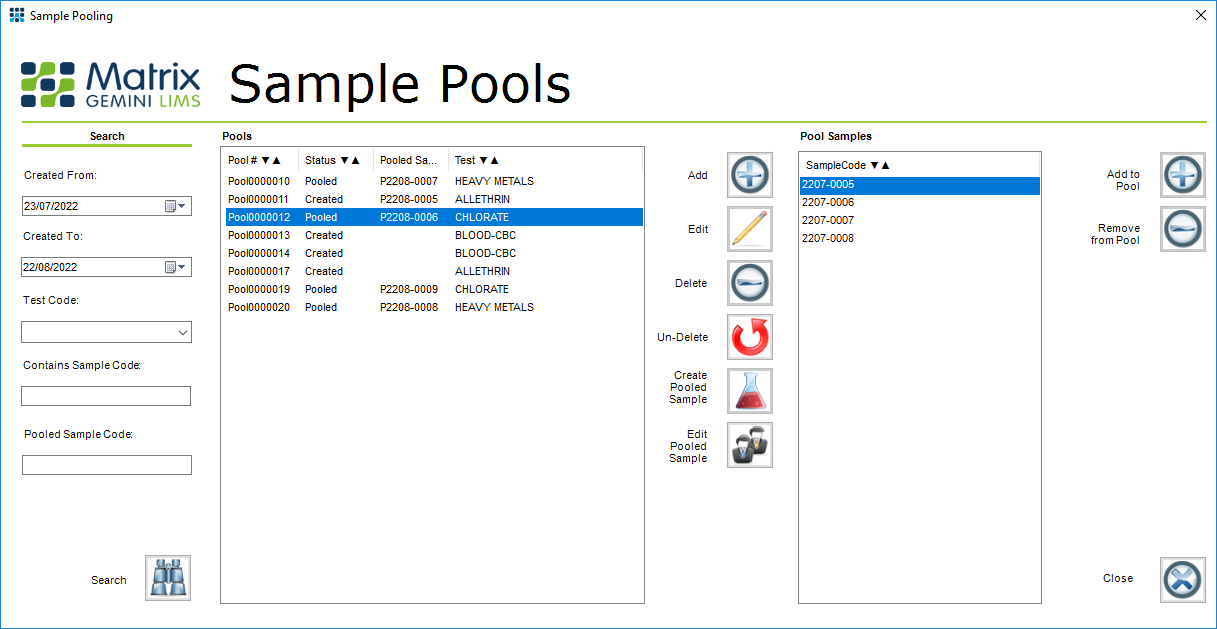
Sample Pooling within a LIMS
Being able to track which samples have been pooled within the LIMS is essential to maintain traceability.
In this sample pooling screen, sample pools are created based on a unique identifier. Samples are then added to the pool as required. In this example the procedure is manual. With high-volume labs, such as those set up for COVID-19 testing, it may be more appropriate to automatically allocate samples to pools. When testing herds or flocks in a veterinary lab it will likely be necessary to ensure that only samples from the same herd or flock are pooled, so that a positive result can be linked to the specific herd.
Given that every screen in Matrix Gemini LIMS is configurable, such criteria can be quickly added as needed to suit the needs of individual laboratories, and to enforce their standard operating procedures. Though not shown in this example, Matrix Gemini LIMS can even be configured to automate initial pooling choices, based on say herd number, and subsequent sub-pooling based on fixed criteria.
To explore LIMS with genuinely flexible pooled sample testing capabilities, please contact us today.
References
1: Sample pooling: burden or solution?, April 17 2021, Grobe et al.
https://www.clinicalmicrobiologyandinfection.com/article/S1198-743X(21)00190-7/fulltext